Structural Evolution of Silica Gel and Silsesquioxane Using Thermal Curing
Abstract
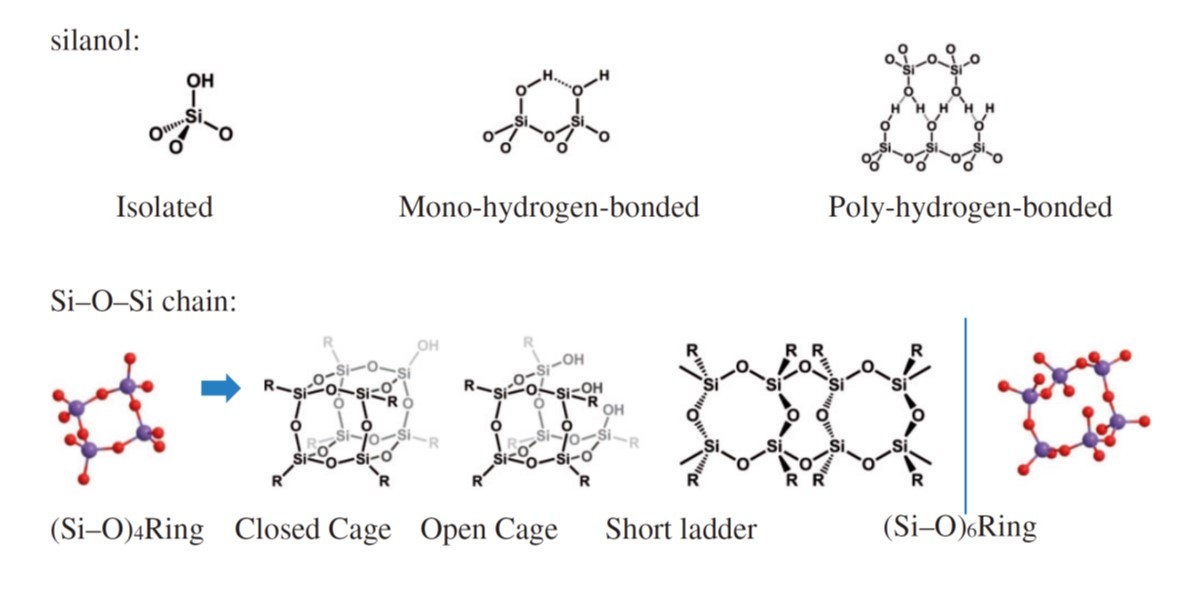
The curing of coatings of two types of siloxane containing materials, silica gel and silsesquioxane, at a modest temperature (<280℃) was studied with in situ heating Fourier transform infrared spectroscopy (FT-IR) in combination with perturbation correlation moving window (PCMW) and two-dimensional correlation spectroscopy (2D-COS) analyses. The result revealed detailed structural evolution of these two different gels. When the silica gel was heated, (Si–O)6 rings appeared from the random Si–O–Si network formed after sol gel reaction, followed by condensation of silanol groups. Upon further heating, the existing (Si–O)4 rings were broken down and converted into (Si–O)6 structures, and finally isolated silanols appeared. The transition from (Si–O)4 rings to (Si–O)6 rings was observed by IR and further confirmed with positron annihilation lifetime spectroscopy (PALS). In comparison, during the curing of hybrid silsesquioxane, the condensation of silanols happens immediately upon heating without the rearrangement of Si–O–Si network. Afterwards, the fraction of (Si–O)6 ring structure increased. (Si–O)4 structures exhibited higher stability in hybrid silsesquioxanes. In addition, the amount of silanols in silsesquioxane continued to reduce without the generation of isolated silanol in the end. The different curing behavior of silsesquioxanes from silica gel originates from the organic groups in silsesquioxanes, which lowers the cross-linking density and reduces the rigidity of siloxane network.
<<全文链接>>

