Antifreezing Hydrogel Electrolyte with Ternary Hydrogen Bonding for High-Performance Zinc-Ion Batteries
Citation
Siwen Huang, Lei Hou, Tianyu Li, Yucong Jiao*, and Peiyi Wu*. Antifreezing Hydrogel Electrolyte with Ternary Hydrogen Bonding for High-Performance Zinc-Ion Batteries. Adv. Mater. 2022, 34, 2110140.
Abstract
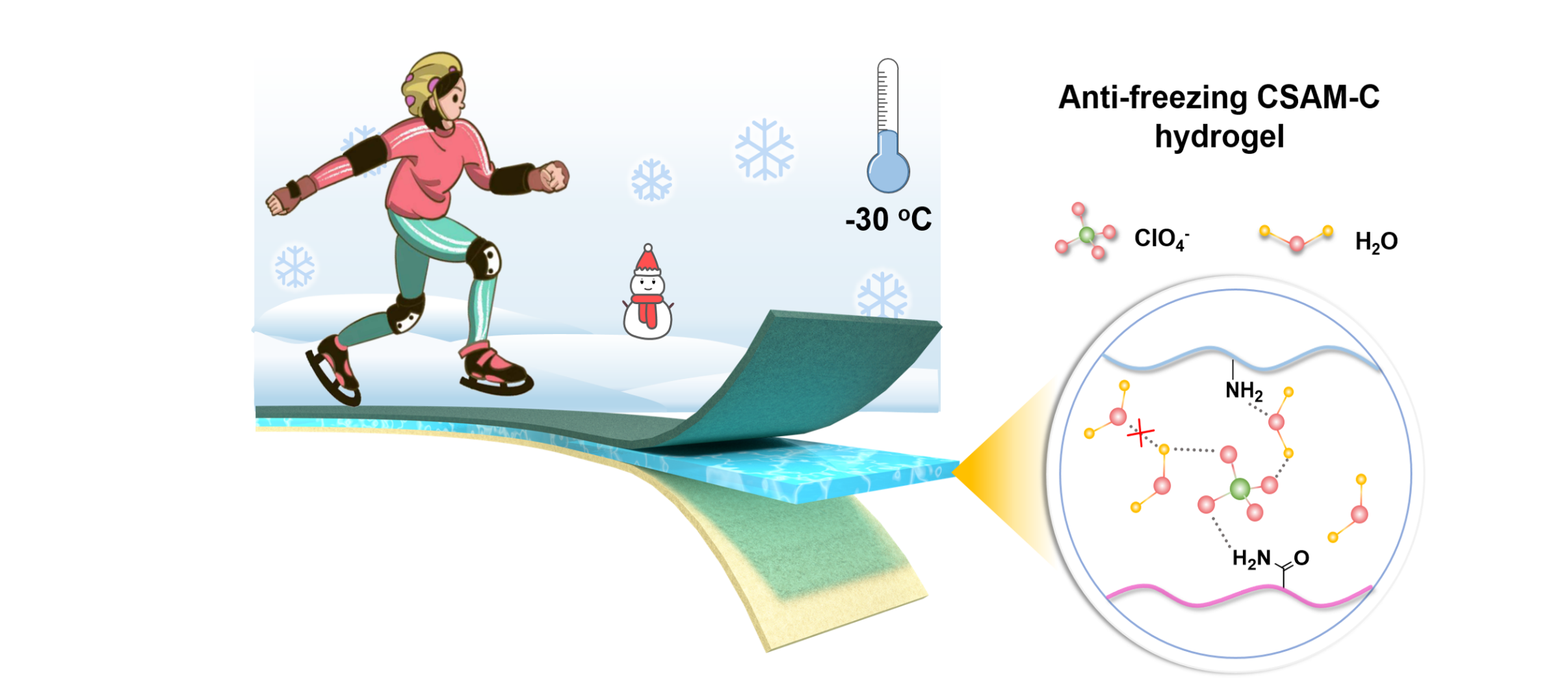
The new-generation flexible aqueous zinc-ion batteries require enhanced mechanical properties and ionic conductivities at low temperature for practical applications. This fundamentally means that it is desired that the hydrogel electrolyte possesses antifreezing merits to resist flexibility loss and performance decrease at subzero temperatures. Herein, a highly flexible polysaccharide hydrogel is realized in situ and is regulated in zinc-ion batteries through the Hofmeister effect with low-concentration Zn(ClO4)2 salts to satisfy the abovementioned requirements. The chaotropic ClO4− anions, water, and polymer chains can form ternary and weak hydrogen bonding (HB), which enables the polymer chains to have improved mechanical properties, breaks the HB of water to remarkably decrease the electrolyte freezing point, and reduces the amounts of free water for effective side reactions and dendrite inhibition. Consequently, even at −30 °C, the Zn(ClO4)2 in situ optimized hydrogel electrolyte features a high ionic conductivity of 7.8 mS cm−1 and excellent flexibility, which enables a Zn/polyaniline (PANI) battery with a reversible capacity of 70 mA h g−1 under 5 A g−1 for 2500 cycles, and renderd the flexible full battery with excellent cycling performances under different bending angles. This work provides a new pathway for designing high-performance antifreezing flexible batteries via the Hofmeister effect.